The attitude towards protecting second medical use claims varies among the ASEAN countries. Thailand has passed explicit guidelines confirming acceptance of second medical use claims. At the same time, Indonesia has skirted around what seemed to be a legislative bar by adopting EPC rules in allowing “product for use” type claims. The latter is an admirably pragmatic approach protecting new indications and possibly a sign of how the next round of patent law reform will shape up in that setting. However, at the other end of the spectrum are Cambodia and Myanmar, in which pharmaceutical patent protection is excluded altogether, and any discussion on second medical use is therefore unnecessary.
As a preliminary point, the overarching principle is that method of medical treatment is specifically excluded from patent protection in these Southeast Asia countries:
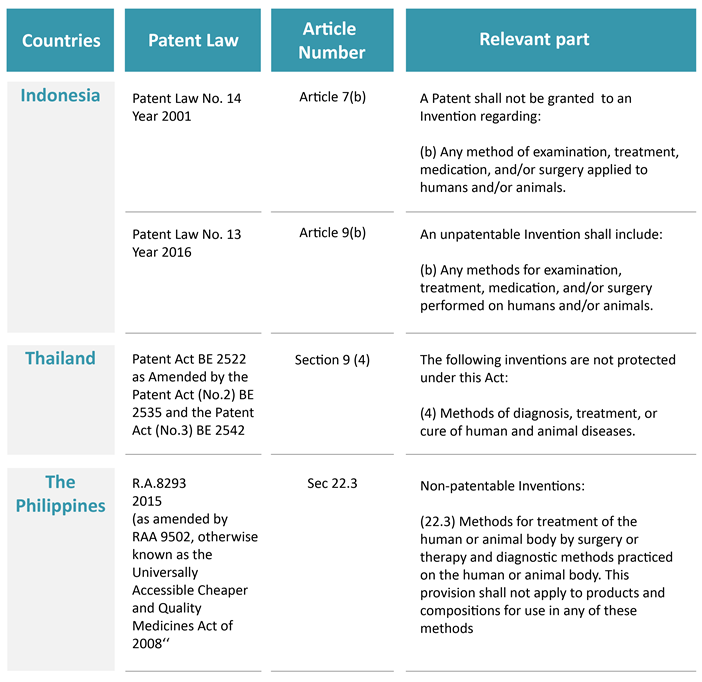
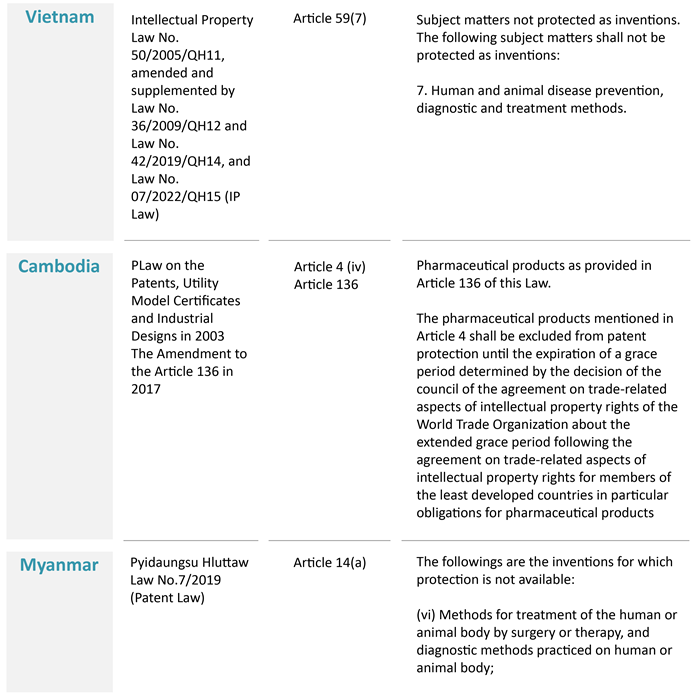
The above, however, does not operate as a bar to patenting new medical indications for pre-existing substances. The following sections discuss specific restrictions against patent protection and how the position has shifted for some Southeast Asian nations.
See article 4(f) of Patent Law No. 13 Year 2016
An Invention shall exclude:
(f) Discovery in the following forms:
1. New use of existing and/or known products;
2. New form of an existing compound that no longer shows improvement in its profound efficacy and has a different chemical structure from the known chemical structure of such compound.
The specific exclusion was introduced with the 2016 revision of the Patent Law.
Before this, Swiss-type claims were accepted in Indonesia Presence of article 7(b) against patenting the treatment method was not an obstacle to Swiss-type claims.
The 2016 Patent Law is under review, and the Directorate General Intellectual Property is considering the removal of this exclusion from the Patent Law.
In the meantime, it is still possible to patent new indications. The Indonesian Patent Office is informally applying Article 54(4)/(5) of the EPC.
However, the Indonesian Patent Office practice may not necessarily be consistent.
The dust has not settled on this because of the apparent conflict with the Indonesian Patent Law article 4(f) mentioned above. Nevertheless, we continue to see grants with the claim format: ‘Substance X used as a medicament for Disease Y‘.
It remains to be seen whether such claims will be open to challenge.
See Section 9 (4) of the Patent Act BE 2522.
‘‘The following inventions are not protected under this Act:
…
(4) methods of diagnosis, treatment or cure of human and animal diseases”
“Thailand’s Patent Act has no specific exclusion for new use of a known substance, but it excludes treatment methods.
The DIP’s 2019 examination guideline confirms that new medical use is potentially patentable if it is not a method of treatment and it meets the requirements of novelty, inventive step, and industrial application capability.
Acceptable claim formats are Swiss-type claims (not necessarily in all circumstances). Also, note that the claims must not directly or indirectly feature dosage or therapeutic steps (including applying the product into a ’subject’s body) that provide efficacy of treatment.
Second medical use is expressly excluded under section 22.1 of the IP Code.
SECTION 22. Non-Patentable Inventions. ‑ The following shall be excluded from patent protection:
22.1. Discoveries, scientific theories, and mathematical methods, and in the case of drugs and medicines, the mere discovery of a new form or new property of a known substance that does not result in the enhancement of the known efficacy of that substance, or the mere discovery of any new property or new use for a known substance, or the mere use of a known process unless such known process results in a new product that employs at least one new reactant.
This exclusion was introduced in 2007 into the IP Code by the passing of the Cheaper Medicines Act.
It was thought the door was shut for second medical use protection in the Philippines. However, in 2018, the IPOHIL revised the Guidelines on the Examination of Pharmaceutical Applications Involving Known Substances (“QUAMA Guide”) – in which references were made to the examination of “new use” of known substances and introduction of the ‘inherency‘ criteria in determining the patentability of such new use, i.e., “a new use of a known substance which is not inherent in the prior art would be a patentable subject matter.”
The commentary in the guideline suggests that the discovery of new use of a known substance is not likely to clear this threshold – new use is not patentable if the discovery of a new use is inherent in the pre-existing substance. In any case, the objection is under section 22.1 IP Code.
The position, as generally understood by practitioners, is that the door is still shut on second medical use patent protection in the Philippines, considering that Section 22.1 was introduced to make medicines affordable, and the guidelines (being a non-legislative instrument) are not likely to change this course.
Point 23.g, Circular No. 16/2016/TT-BKHCN (effective 15 August 2018) settles the position shutting the door to second medical use claims. See below said provision.
Point 23.g, Circular No. 16/2016/TT-BKHCN became effective 15 August 2018 (“Circular 16”):
‘’…function/utility of a claimed subject matter is not an essential technical feature, but may be the purpose, result obtained of such subject matter.”
Point 23.g rules out claim over new indication as “not containing essential technical feature”.
Before the Circular, the Vietnam patent office suspended applications containing medical use claim. The Circular provided a basis for rejecting the suspended applications, which the Patent Office did.
Myanmar and Cambodia are still not obligated to grant patent protection to pharmaceutical research products until 2033 because of waivers issued by the WTO’s Council for Trade-Related Aspects of Intellectual Property Rights (TRIPS). In the meantime, Cambodia has a mailbox system to receive pharma applications to preserve their respective priorities.
As seen above, the position on protecting new indications varies because of different stages of economic development and differences in dealing with pharmaceutical accessibility.